ivWatch, a company based in Newport News, Virginia, won FDA clearance for its SmartTouch sensor that detects peripheral IV infiltration and extravasation events. Though somewhat rare, these can be difficult to notice and a late response can lead to grave consequences. The SmartTouch sensor can warn physicians that something is wrong, sometimes hours before a clinician would notice any visual or tactile changes on the patient’s body.
The sensor, which is already cleared in Europe, is a single-use disposable device that can be used on patients of any age, including those in the neonatal intensive care unit. Thanks to the SmartTouch, clinicians can take advantage of additional options in terms of where to place the IV, which is especially important for neonates, to choose longer dwell times, and to keep an eye on active patients that may be ambulatory at times.
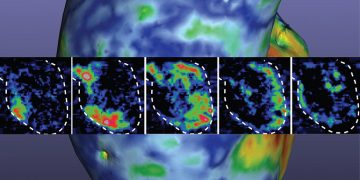
The device, which uses visible and near-infrared light to penetrate the skin, can be used alongside the ivWatch Patient Monitor that algorithmically analyzes the optical signatures of tissue around the IV site while compensating for patient movements. The system can detect changes as small as .2 mL of IV fluid, with an average detection volume of 2.02 mL, according to ivWatch. Any adverse events are immediately relayed to the clinical team for a quick response.
The adhesive on the back side of the sensor is breathable, leading to minimal irritation and compatibility with existing IV dressings.

More from ivWatch regarding the tested efficacy of the SmartTouch:
A series of seven IRB-approved studies were conducted to test the efficacy and safety of the SmartTouch Sensor. Five verification studies were conducted to understand the performance and optimize ivWatch’s proprietary algorithm to maximize sensitivity to infiltrated tissues, while limiting the number of false notifications issued. Two validation studies were conducted to investigate device sensitivity and false notification rates.
Clinical data shows that the SmartTouch Sensor issued notifications for 99.0%* of early stage infiltrations in less than 10 mL of infused IV fluid. Results also showed less than one false notification was issued every six days, therefore having minimal contribution to clinician alarm fatigue.[3],[4],[5] The majority of the non-infiltration notifications were attributed to a force applied to the IV site, which may beneficially notify clinicians of conditions that could place the peripheral IV site at a greater risk for complications. ivWatch also has a proven record of detecting IV infiltrations an average of 15 hours before the clinician.[2]
Flashbacks: ivWatch Detects IV Infiltrations and Extravasations: Interview with Gary Warren, President and CEO; ivWatch Monitors IV Placement Sites for Leakage Outside Veins; ivWatch IV Infiltration Detection Device Cleared in Europe; ivWatch Detects Leaks Near IV Placement Sites;
Product page: SmartTouch Sensor
Via: ivWatch