To monitor, treat, and better understand how seizures arise and develop, scientists and clinicians have been looking for more objective measures and analyses of brain activity. Currently, a typical electroencephalography (EEG) recording of brainwaves throughout a seizure is of limited value as it requires quite a bit of processing to get a sense of what happened. A team of researchers at Washington University in St. Louis, University of Texas at Arlington, and Northeastern University have combined forces to develop a new computing approach that more intelligently crunches the data so that oncoming seizures, and their unique features, can be quantified in real time.
“Our technique allows us to get raw data, process it and extract a feature that’s more informative for the machine learning model to use,” said Walter Bomela, one of the scientists, in the announcement. “The major advantage of our approach is to fuse signals from 23 electrodes to one parameter that can be efficiently processed with much less computing resources.”

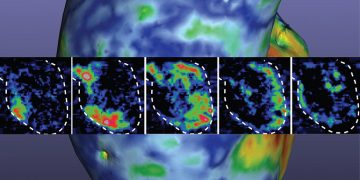
Caption: This gif was recorded during two seizures, one at 2950 seconds, the other at 9200. The top left animation is of EEG signals from three electrodes. The top right is a map of the inferred network. The third animation plots the Fiedler eigenvalue, the single value used to detect seizures using the network inference technique. (Courtesy: Walter Bomela, Li Lab)
A simple glance at an EEG readout during a seizure shows normal brain activity turning to strong, synchronized discharges that overwhelm much of the brain. These are believed to arise from groups of neurons that seem to want to act together and amplify each other. “But the seizure detection accuracy is not that good when temporal EEG signals are used,” says Bomela.
To get a more nuanced understanding of the data, and spot where and when seizures are arising, an algorithm based on network inference was developed by the research team. “We treated EEG electrodes as nodes of a network. Using the recordings (time-series data) from each node, we developed a data-driven approach to infer time-varying connections in the network or relationships between nodes,” Bomela says. “We want to infer how a brain region is interacting with others,” he added.
The algorithm, which so far has been trained to work with a single individual, quickly identifies signal networks and analyzes its various parameters. One such is the Fiedler eigenvalue, which measures the synchronicity of a network, and which has shown to be of particular interest as it rises with the onset of a seizure. Moreover, the same parameter helps to minimize signal noise and distractions from stray signals originating from normal body activity.
The researchers are now planning on expanding this technique to work with other people suffering from epilepsy and quantify and evaluate different types of seizures.
Study in journal Scientific Reports: Real-time Inference and Detection of Disruptive EEG Networks for Epileptic Seizures